Insider Brief
- Vitestro reports that all performance and safety endpoints for its autonomous blood drawing devices were met in a diverse, outpatient study population.
- The study is considered the world’s largest evaluation of autonomous blood drawing devices.
- Critical Quote: “Blood draws are a critical aspect of daily lab operations and the most common invasive medical procedure in all of medicine, yet little about the procedure has changed in the past half-century.” — Thijs van Holten, Ph.D., Clinical Chemist at St. Antonius Hospital, Nieuwegein
- Image: Vitestro
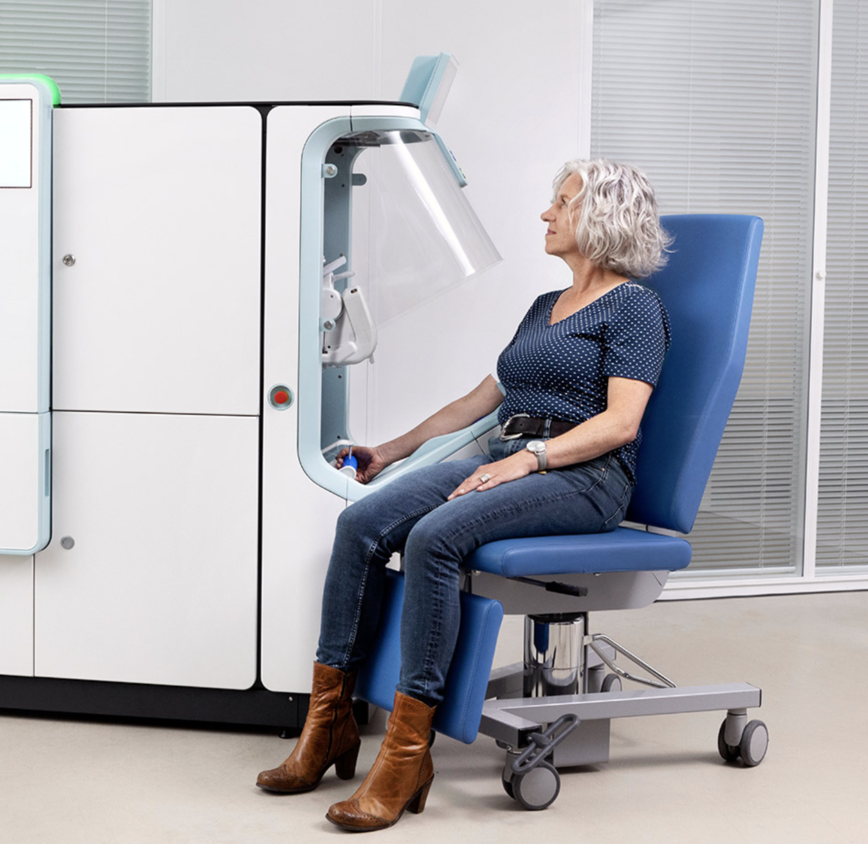
PRESS RELEASE — Vitestro, the pioneering autonomous blood drawing company, today announced that Pivotal Trial results from its Autonomous Blood Drawing Optimization and Performance Testing (A.D.O.P.T.) study were shared at IFCC WorldLab 2024 in Dubai. The study is the world’s largest evaluation of autonomous blood drawing devices.
The two-year A.D.O.P.T. study (NCT05878483) is scheduled to run through 2025 and involves 10,000 patients over five study phases. The study sites include four leading Dutch hospital labs: St. Antonius Hospital, OLVG Lab, Result Laboratorium, and Amsterdam UMC.
In the now-completed EU Pivotal Trial phase, all performance and safety endpoints were met in a diverse, outpatient study population (age range: 18-92 years; 20% of the study population was difficult to puncture). Furthermore, results from the follow-on study phase show that Vitestro’s technology achieved 95% first-stick success.
Key findings from the study include:
– Ninety-eight percent (98%) of patients indicated acceptance of the new, automated method of blood drawing
– Eighty-three percent (83%) of patients rated the procedure pain as less than or comparable to – manual blood draws
– Median time of procedure was 1 minute and 49 seconds
– In vitro hemolysis rate for collected blood samples was 0.6%
– No serious or moderate adverse events occurred
Vitestro’s 95% first-stick success is in line with traditional manual venipuncture, for which average first-stick success rates of 93–97% have been reported, although performance can be as low as 80-89% for manual venipuncture depending on vein difficulty and the phlebotomist.
The 0.6% hemolysis rate also meets clinical practice standards. According to the American Society of Clinical Pathology, the benchmark for hemolysis rates is less than or equal to 2%.
For more information about the study results, please see this link.
“Blood draws are a critical aspect of daily lab operations and the most common invasive medical procedure in all of medicine, yet little about the procedure has changed in the past half-century,” said Thijs van Holten, Ph.D., Clinical Chemist at St. Antonius Hospital, Nieuwegein, The Netherlands and one of the study’s Principal Investigators. “These study results clearly demonstrate the potential for this new way of managing blood draws using advanced technologies.”
Through a combination of advanced imaging technology and robotics, the Vitestro device enables hospitals and labs to provide accurate and autonomous blood draws, reducing the need for manual handling and improving patient and clinician satisfaction. This is critical as the medical laboratory market is experiencing a severe staffing shortage that has left labs challenged to delivery timely test results and consistent patient care.
The Vitestro technology continues to generate excitement from patients and health systems. Several Vitestro devices have already been pre-ordered and are scheduled to be deployed at European hospitals later this year to help ease workload and provide a more consistent, improved patient experience.
The company is on track to obtain CE marking by the end of 2024 and just announced a $22 million funding round to accelerate its commercialization work, bringing its total funding raised to $50 million.
“These study results confirm the performance and safety of the autonomous blood draw device and will be used to support EU regulatory approval,” said Luuk Giesen, M.D., Coordinating Investigator and Chief Medical Officer at Vitestro. “We’re excited to continue with the A.D.O.P.T. trial and expand the reach of this revolutionary device globally as we define the future standard of care for blood draws.”
For more information about Vitestro and its innovative blood drawing device, visit https://vitestro.com.